Dynamics of confined water and its interplay with alkali cations in sodium aluminosilicate hydrate gel: insights from reactive force field molecular dynamics†
Abstract
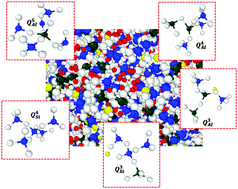
This paper presents the dynamics of confined water and its interplay with alkali cations in disordered sodium aluminosilicate hydrate (N-A-S-H) gel using reactive force field molecular dynamics. N-A-S-H gel is the primary binding phase in geopolymers formed via alkaline activation of fly ash. Despite attractive mechanical properties, geopolymers suffer from durability issues, particularly the alkali leaching problem which has motivated this study. Here, the dynamics of confined water and the mobility of alkali cations in N-A-S-H is evaluated by obtaining the evolution of mean squared displacements and Van Hove correlation function. To evaluate the influence of the composition of N-A-S-H on the water dynamics and diffusion of alkali cations, atomistic structures of N-A-S-H with Si/Al ratio ranging from 1 to 3 are constructed. It is observed that the diffusion of confined water and sodium is significantly influenced by the Si/Al ratio. The confined water molecules in N-A-S-H exhibit a multistage dynamic behavior where they can be classified as mobile and immobile water molecules. While the mobility of water molecules gets progressively restricted with an increase in Si/Al ratio, the diffusion coefficient of sodium also decreases as the Si/Al ratio increases. The diffusion coefficient of water molecules in the N-A-S-H structure exhibits a lower value than those of the calcium-silicate-hydrate (C-S-H) structure. This is mainly due to the random disordered structure of N-A-S-H as compared to the layered C-S-H structure. To further evaluate the influence of water content in N-A-S-H, atomistic structures of N-A-S-H with water contents ranging from 5–20% are constructed. Qn distribution of the structures indicates significant depolymerization of N-A-S-H structure with increasing water content. Increased conversion of Si–O–Na network to Si–O–H and Na–OH components with an increase in water content helps explain the alkali-leaching issue in fly ash-based geopolymers observed macroscopically. Overall, the results in this study can be used as a starting point towards multiscale simulation-based design and development of durable geopolymers.


 Please wait while we load your content...
Please wait while we load your content...