Substitution effect on the nonradiative decay and trans → cis photoisomerization route: a guideline to develop efficient cinnamate-based sunscreens†
Abstract
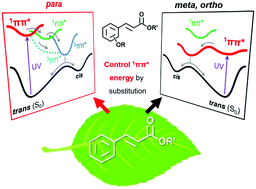
Cinnamate derivatives are very useful as UV protectors in nature and as sunscreen reagents in daily life. They convert harmful UV energy to thermal energy through effective nonradiative decay (NRD) including trans → cis photoisomerization. However, the mechanism is not simple because different photoisomeirzation routes have been observed for different substituted cinnamates. Here, we theoretically examined the substitution effects at the phenyl ring of methylcinnamate (MC), a non-substituted cinnamate, on the electronic structure and the NRD route involving trans → cis isomerization based on time-dependent density functional theory. A systematic reaction pathway search using the single-component artificial force-induced reaction method shows that the very efficient photoisomerization route of MC can be essentially described as “1ππ* (trans) → 1nπ* → T1 (3ππ*) → S0 (trans or cis)”. We found that for efficient 1ππ* (trans) → 1nπ* internal conversion (IC), MC should have the substituent at the appropriate position of the phenyl ring to stabilize the highest occupied π orbital. Substitution at the para position of MC slightly lowers the 1ππ* state energy and photoisomerization occurs via a slightly less efficient “1ππ* (trans) → 3nπ* → T1 (3ππ*) → S0 (trans or cis)” pathway. Substitution at the meta or ortho positions of MC significantly lowers the 1ππ* state energy so that the energy barrier of IC (1ππ* → 1nπ*) becomes very high. This substitution leads to a much longer 1ππ* state lifetime than that of MC and para-substituted MC, and a change in the dominant photoisomerization route to “1ππ* (trans) → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond twisting on 1ππ* → S0 (trans or cis)”. As a whole, the “1ππ* → 1nπ*” IC observed in MC is the most important initial step for the rapid change of UV energy to thermal energy. We also found that the stabilization of the π orbital (i) minimizes the energy gap between 1ππ* and 1nπ* at the 1ππ* minimum and (ii) makes the 0–0 level of 1ππ* higher than 1nπ* as observed in MC. These MC-like relationships between the 1ππ* and 1nπ* energies should be ideal to maximize the “1ππ* → 1nπ*” IC rate constant according to Marcus theory.
C bond twisting on 1ππ* → S0 (trans or cis)”. As a whole, the “1ππ* → 1nπ*” IC observed in MC is the most important initial step for the rapid change of UV energy to thermal energy. We also found that the stabilization of the π orbital (i) minimizes the energy gap between 1ππ* and 1nπ* at the 1ππ* minimum and (ii) makes the 0–0 level of 1ππ* higher than 1nπ* as observed in MC. These MC-like relationships between the 1ππ* and 1nπ* energies should be ideal to maximize the “1ππ* → 1nπ*” IC rate constant according to Marcus theory.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...