Redox potentials along the redox-active low-barrier H-bonds in electron transfer pathways†
Abstract
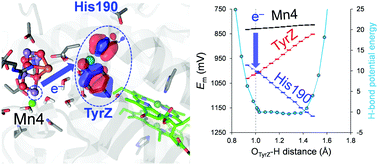
Low-barrier H-bonds form when the pKa values of the H-bond donor and acceptor moieties are nearly equal. Here, we report redox potential (Em) values along two redox-active low-barrier H-bonds in the water-oxidizing enzyme photosystem II (PSII), using a quantum mechanical/molecular mechanical approach. The low-barrier H-bond between D1-Tyr161 (TyrZ) and D1-His190 is located in the middle of the electron transfer pathway. When the proton is at D1-His190, Em(TyrZ) is the lowest and can serve as an electron donor to the oxidized chlorophyll PD1˙+. Em(TyrZ) and Em(D1-His190) are equal, and the TyrZ⋯D1-His190 pair serves as an electron acceptor to Mn4CaO5 when the proton is at TyrZ. In the low-barrier H-bond between D1-His215 and plastoquinone QB, located at the terminus of the electron transfer pathway, the driving force of electron transfer and electronic coupling between QA and QB are maximized when the proton arrives at QB. It seems likely that local proton transfer along redox-active low-barrier H-bonds can alter the driving force or electronic coupling for electron transfer.


 Please wait while we load your content...
Please wait while we load your content...