Thermal dehydration of calcium sulfate dihydrate: physico-geometrical kinetic modeling and the influence of self-generated water vapor†
Abstract
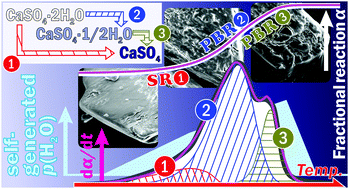
Complex kinetic behaviors in the thermal dehydration of CaSO4·2H2O under varying water vapor pressure (p(H2O)) conditions impel researchers in the field of solid-state kinetics to gain a more comprehensive understanding. Both self-generated and atmospheric p(H2O) are responsible for determining the reaction pathways and the overall kinetic behaviors. This study focuses on the influence of the self-generated water vapor to obtain further insights into the complexity of the kinetic behaviors. The single-step mass-loss process under conditions generating a low p(H2O) was characterized kinetically by a physico-geometrical consecutive induction period, surface reaction, and phase boundary-controlled reaction, along with the evaluation of the kinetic parameters for the individual physico-geometrical reaction steps. Under the conditions in which more p(H2O) was generated, the overall reaction to form the anhydride was interpreted as a three-step process, comprising the initial reaction (direct dehydration to the anhydride) and a subsequent two-step reaction via the intermediate hemihydrate, which was caused by the variations in the self-generated p(H2O) conditions as the reaction advanced. The variations in the reaction pathways and kinetics behaviors under the self-generated p(H2O) conditions are discussed through a systematic kinetic analysis conducted using advanced kinetic approaches for the multistep process.


 Please wait while we load your content...
Please wait while we load your content...