Hydrogenated polycyclic aromatic hydrocarbons: isomerism and aromaticity
Abstract
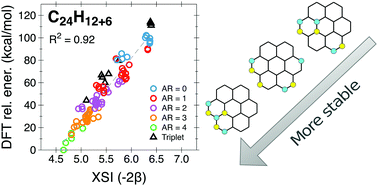
A simple model, based on connectivity (adjacency) matrices, is introduced to study the relative stability of hydrogenated polycyclic aromatic hydrocarbons (HPAHs). The model allows us to consider a very large number of isomeric structures for HPAHs of variable size and degree of hydrogenation, by taking into account the different positions available in each hydrogenation step. The validity of our approach is demonstrated by comparing, for a few selected cases, with the predictions of Density Functional Theory calculations. We have found that aromaticity is the main factor governing the relative stability of HPAH isomers and that the most stable structures are in general those containing the maximum possible number of non-hydrogenated rings.


 Please wait while we load your content...
Please wait while we load your content...