Solvent-dependent termination, size and stability in polyynes synthesized via laser ablation in liquids†
Abstract
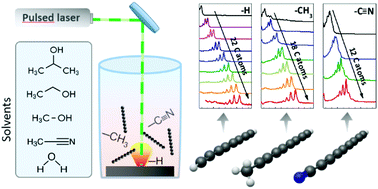
In recent years there has been growing interest in sp-carbon chains as possible novel nanostructures. An example of sp-carbon chains is the so-called polyyne, characterized by the alternation of single and triple bonds that can be synthesized via pulsed laser ablation in liquid (PLAL) of a graphite target. In this work, by using different solvents in the PLAL process, e.g. water, acetonitrile, methanol, ethanol, and isopropanol, we systematically investigated the role of the solvent in polyyne synthesis and stability, and discussed the possible formation mechanisms. The presence of methyl- and cyano-groups in the solutions influences the termination of polyynes, allowing the detection, of hydrogen-capped polyynes up to H-C22-H, methyl-capped polyynes up to H-C18-CH3 and cyanopolyynes up to H-C12-CN. The assignment of each species was performed via UV-vis spectroscopy and supported by density functional theory simulations of vibronic spectra. In addition, surface-enhanced Raman spectroscopy allowed to highlight the differences in the shape and positions of the characteristic Raman bands of the size-selected polyynes with different terminations (hydrogen, methyl and cyano groups). The stability in time of each polyyne was investigated by evaluating the chromatographic peak area, and the effect of size, terminations and solvents on polyyne stability was individuated.


 Please wait while we load your content...
Please wait while we load your content...