Achieving tunable chemical reactivity through photo-initiation of energetic materials at metal oxide surfaces†
Abstract
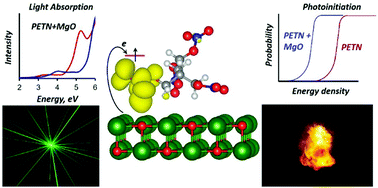
Known applications of high energy density materials are impressively vast. Despite this, we argue that energetic materials are still underutilized for common energy purposes due to our inability to control explosive chemical reactions releasing energy from these materials. The situation appears paradoxical as energetic materials (EM) possess massive amounts of energy and, hence, should be most appropriate for applications in many energy-intensive processes. Here, we discover how chemical decomposition reactions can be stimulated with laser excitation and therefore, highly controlled by selectively designing energetic material – metal oxide interfaces with an example of pentaerythritol tetranitrate (PETN)–MgO and trinitrotoluene (TNT)–MgO composite samples. Density functional theory and embedded cluster method calculations were combined with measurements of the optical absorption spectra and laser initiation experiments. We found that the first (1064 nm, 1.17 eV), second (532 nm, 2.33 eV), and third (355 nm, 3.49 eV) laser harmonics, to all of which pure energetic materials are transparent, can be effectively used to trigger explosive reactions in the PETN–MgO samples. We propose a consistent electronic mechanism that explains how specific sub-band optical transitions initiate decomposition chemistry. Also, this selectivity reveals a fundamental difference between materials chemistry at interfaces as we show on examples of PETN and TNT energetic materials.


 Please wait while we load your content...
Please wait while we load your content...