Synergistic effect of two action sites on a nitrogen-doped carbon catalyst towards acetylene hydrochlorination†
Abstract
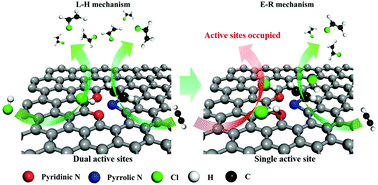
Whether the reaction pathway is steady or dynamic over the whole life cycle of a catalyst process can facilitate our understanding of its catalytic behavior. Herein, the dynamic reaction pathways of nitrogen-doped carbon catalysts are investigated in acetylene hydrochlorination. When triggered, the reaction follows the Langmuir–Hinshelwood mechanism with pyrrolic N and pyridinic N as dual active sites. However, pyridinic N is deactivated first, due to the strong adsorption of hydrogen chloride, causing the reaction to further run with pyrrolic N as the single active site and follow the Eley–Rideal mechanism. This work provides a new promising way to study the catalytic behavior of nitrogen-doped carbon catalysts.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...