Unsaturated binuclear homoleptic nickel carbonyl anions Ni2(CO)n− (n = 4–6) featuring double three-center two-electron Ni–C–Ni bonds†
Abstract
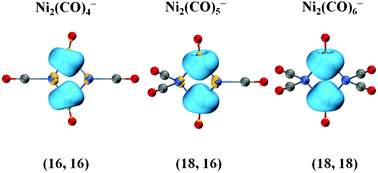
The homoleptic homodinuclear nickel carbonyl anions Ni2(CO)n− (n = 4–6) are mass-selected in the gas phase and examined with anion photoelectron velocity-map imaging spectroscopy combined with density functional calculations. The doubly carbonyl-bridged structures are found to be favorable for Ni2(CO)n− (n = 4–6). The nature of Ni–Ni bonding in these complexes is analysed with the aid of a range of state-of-the-art quantum chemistry methods. Despite the absence of direct multiple Ni–Ni bonds, the two nickel atoms in Ni2(CO)n− (n = 4–6) complexes are joined by two bridging carbonyl ligands via the sharing three-center two-electron Ni–C–Ni bond in turn to achieve the (16,16), (16,18), and eventually the favored (18,18) configurations.


 Please wait while we load your content...
Please wait while we load your content...