Gas phase formation of cyclopentanaphthalene (benzindene) isomers via reactions of 5- and 6-indenyl radicals with vinylacetylene†
Abstract
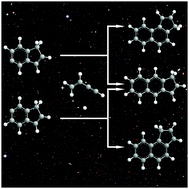
The tricyclic polycyclic aromatic hydrocarbons (PAHs) 3H-cyclopenta[a]naphthalene (C13H10), 1H-cyclopenta[b]naphthalene (C13H10) and 1H-cyclopenta[a]naphthalene (C13H10) along with their indene-based bicyclic isomers (E)-5-(but-1-en-3-yn-1-yl)-1H-indene, (E)-6-(but-1-en-3-yn-1-yl)-1H-indene, 5-(but-3-ene-1-yn-1-yl)-1H-in-dene, and 6-(but-3-ene-1-yn-1-yl)-1H-indene were formed via a “directed synthesis” in a high-temperature chemical micro reactor at the temperature of 1300 ± 10 K through the reactions of the 5- and 6-indenyl radicals (C9H7˙) with vinylacetylene (C4H4). The isomer distributions were probed utilizing tunable vacuum ultraviolet light by recording the photoionization efficiency curves at mass-to-charge of m/z = 166 (C13H10) and 167 (13CC12H10) of the products in a supersonic molecular beam. The underlying reaction mechanisms involve the initial formation of van-der-Waals complexes followed by addition of the 5- and 6-indenyl radicals to vinylacetylene via submerged barriers, followed by isomerization (hydrogen shifts, ring closures), and termination via atomic hydrogen elimination accompanied by aromatization. All the barriers involved in the formation of 3H-cyclopenta[a]naphthalene, 1H-cyclopenta[b]naphthalene and 1H-cyclopenta[a]naphthalene are submerged with respect to the reactants indicating that the mechanisms are in fact barrierless, potentially forming PAHs via the hydrogen abstraction – vinylacetylene addition (HAVA) pathway in the cold molecular clouds such as Taurus Molecular Cloud-1 (TMC-1) at temperatures as low as 10 K.


 Please wait while we load your content...
Please wait while we load your content...