Solvatochromism in urea/water and urea-derivative/water solutions†
Abstract
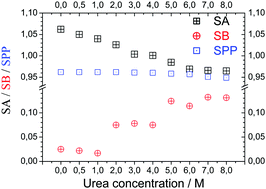
This work reports the experimental measurements of solvent acidity (SA), basicity (SB), and solvent dipolarity and polarizability (SPP) for water solutions with urea (U) and its molecular derivatives, monomethyl-urea (MU), 1,3-dimethyl-urea (DMU) and tetramethyl-urea (TMU). These solvatochromic parameters are applied to understanding the variation of indexes of refraction and densities and other physico-chemical properties reported for these solutions. These properties are well correlated to the SA, SB, and SPP solvent parameters of these solutions. As a result, from the characterization of the physico-chemical properties, one can infer that urea and its molecular derivatives are mainly modifiers in the structure of liquid water. The solvatochromic parameters indicate the possible existence of different mechanisms in the denaturation process of proteins in these urea/water solutions.


 Please wait while we load your content...
Please wait while we load your content...