Effects of paramagnetic fluctuations on the thermochemistry of MnO(100) surfaces in the oxygen evolution reaction†
Abstract
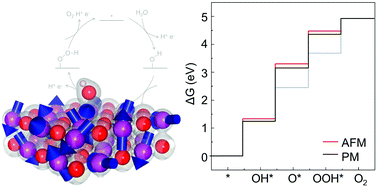
We investigated the effects of paramagnetic (PM) fluctuations on the thermochemistry of the MnO(100) surface in the oxygen evolution reaction (OER) using the “noncollinear magnetic sampling method plus U” (NCMSM+U). Various physical properties, such as the electronic structure, free energy, and charge occupation, of the MnO(100) surface in the PM state with several OER intermediates, were reckoned and compared to those in the antiferromagnetic (AFM) state. We found that PM fluctuation enhances charge transfer from a surface Mn ion to each of the intermediates and strengthens the chemical bond between them, while not altering the overall features, such as the rate determining step and resting state, in reaction pathways. The enhanced charge transfer can be attributed to the delocalized nature of valence bands observed in the PM surface. In addition, it was observed that chemical-bond enhancement depends on the intermediates, resulting in significant deviations in reaction energy barriers. Our study suggests that PM fluctuations play a significant role in the thermochemistry of chemical reactions occurring on correlated oxide surfaces.


 Please wait while we load your content...
Please wait while we load your content...