Vibrational energy relaxation of interfacial OH on a water-covered α-Al2O3(0001) surface: a non-equilibrium ab initio molecular dynamics study
Abstract
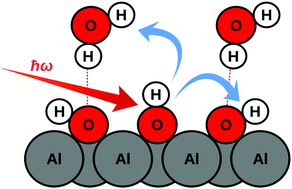
Vibrational relaxation of adsorbates is a sensitive tool to probe energy transfer at gas/solid and liquid/solid interfaces. The most direct way to study relaxation dynamics uses time-resolved spectroscopy. Here we report on a non-equilibrium ab initio molecular dynamics (NE-AIMD) methodology to model vibrational relaxation of OH vibrations on a hydroxylated, water-covered α-Al2O3(0001) surface. In our NE-AIMD approach, after exciting selected O–H bonds their coupling to surface phonons and to the water adlayer is analyzed in detail, by following both the energy flow in time, as well as the time-evolution of Vibrational Density of States (VDOS) curves. The latter are obtained from Time-dependent Correlation Functions (TCFs) and serve as prototypical, generic representatives of time-resolved vibrational spectra. As most important results, (i) we find a few-picosecond lifetime of the excited modes and (ii) identify both hydrogen-bonded aluminols and water molecules in the adsorbed water layer as main dissipative channels, while the direct coupling to Al2O3 surface phonons is of minor importance on the timescales of interest. Our NE-AIMD/TCF methodology is powerful for complex adsorbate systems, in principle even reacting ones, and opens a way towards time-resolved vibrational spectroscopy.
- This article is part of the themed collection: Festschrift for Peter Toennies - New Horizons in the Dynamics of Molecules: from Gases to Surfaces


 Please wait while we load your content...
Please wait while we load your content...