Adsorption and reactions of NO molecules on anionic gold clusters in the size range below 1 nm: effects of clusters’ global electronic properties†
Abstract
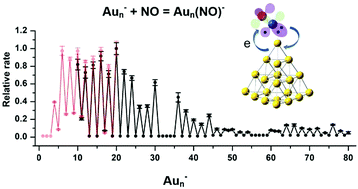
We systemically studied adsorption and reactions of NO on Aun− (n ≤ 80) using a mini flow-tube reactor running at 150 K. For Aun− (n ≤ 11), their reactions with NO mainly formed cluster complexes containing various numbers of NO units; for Aun− (n ≥ 12), most active sizes eventually formed specific complexes Aun(NO)3−. The relative rates of the reactions with the first NO were measured. Correlations between these relative rates and the adiabatic detachment energies (ADEs) of Aun− revealed the dominant effect of the clusters’ spins and a more complicated electron transfer mechanism than that of reactions with O2. Au20− as well as previously reported Au4,6,8− is an exceptional size, which eventually formed the disproportionate product Au20NO2−, and all these four sizes have very low ADEs. The effects of the clusters’ global electronic properties on adsorption and reactions of NO on anionic gold are helpful to understand catalytic mechanisms of gold-based catalysts in NO removal reactions.


 Please wait while we load your content...
Please wait while we load your content...