Charge regulation of colloidal particles in aqueous solutions
Abstract
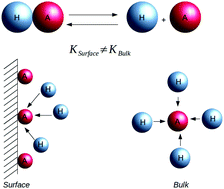
We study the charge regulation of colloidal particles inside aqueous electrolyte solutions. To stabilize a colloidal suspension against precipitation, colloidal particles are synthesized with either acidic or basic groups on their surface. On contact with water, these surface groups undergo proton transfer reactions, resulting in colloidal surface charge. The charge is determined by the condition of local chemical equilibrium between hydronium ions inside the solution and at the colloidal surface. We use a model of Baxter sticky spheres to explicitly calculate the equilibrium dissociation constants and to construct a theory which is able to quantitatively predict the effective charge of colloidal particles with either acidic or basic surface groups. The predictions of the theory for the model are found to be in excellent agreement with the results of Monte Carlo simulations. This theory is further extended to treat colloidal particles with a mixture of both acidic and basic surface groups.


 Please wait while we load your content...
Please wait while we load your content...