Hydrogen in methanol catalysts by neutron imaging
Abstract
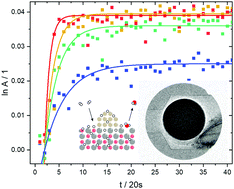
Although of pivotal importance in heterogeneous hydrogenation reactions, the amount of hydrogen on catalysts during reactions is seldom known. We demonstrate the use of neutron imaging to follow and quantify hydrogen containing species in Cu/ZnO catalysts operando during methanol synthesis. The steady-state measurements reveal that the amount of hydrogen containing intermediates is related to the reaction yields of CO and methanol, as expected from simple considerations of the likely reaction mechanism. The time-resolved measurements indicate that these intermediates, despite indispensable within the course of the reaction, slow down the overall reaction steps. Hydrogen–deuterium exchange experiments indicate that hydrogen reduction of Cu/ZnO nano-composites modifies the catalyst in such a way that at operating temperatures, hydrogen is dynamically absorbed in the ZnO-nanoparticles. This explains the extraordinary good catalysis of copper if supported on ZnO by its ability to act as a hydrogen reservoir supplying hydrogen to the surface covered by CO2, intermediates, and products during catalysis.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...