Bifunctional catalytic activity of Zn1−xFexO toward the OER/ORR: seeking an optimal stoichiometry
Abstract
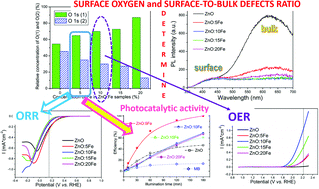
Eco-friendly and rapid microwave processing of a precipitate was used to produce Fe-doped zinc oxide (Zn1−xFexO, x = 0, 0.05, 0.1, 0.15 and 0.20; ZnO:Fe) nanoparticles, which were tested as catalysts toward the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) in a moderately alkaline solution. The phase composition, crystal structure, morphology, textural properties, surface chemistry, optical properties and band structure were examined to comprehend the influence of Zn2+ partial substitution with Fe3+ on the catalytic activity of ZnO:Fe. Linear sweep voltammetry showed an improved catalytic activity of ZnO:5Fe toward the ORR, compared to pure ZnO, while with increased amounts of the Fe-dopant the activity decreased. The improvement was suggested by a more positive onset potential (0.394 V vs. RHE), current density (0.231 mA cm−2 at 0.150 V vs. RHE), and faster kinetics (Tafel slope, b = 248 mV dec−1), and it may be due to the synergistic effect of (1) a sufficient amount of surface oxygen vacancies, and (2) a certain amount of plate-like particles composed of crystallites with well developed (0001) and (000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) facets. Quite the contrary, the OER study showed that the introduction of Fe3+ ions into the ZnO crystal structure resulted in enhanced catalytic activity of all ZnO:Fe samples, compared to pure ZnO, probably due to the modified binding energy and an optimized band structure. With the maximal current density of 1.066 mA cm−2 at 2.216 V vs. RHE, an onset potential of 1.856 V vs. RHE, and the smallest potential difference between the OER and ORR (ΔE = 1.58 V), ZnO:10Fe may be considered a promising bifunctional catalyst toward the OER/ORR in moderately alkaline solution. This study demonstrates that the electrocatalytic activity of ZnO:Fe strongly depends on the defect chemistry and consequently the band structure. Along with providing fundamental insight into the electrocatalytic activity of ZnO:Fe, the study also indicates an optimal stoichiometry for enhanced bifunctional activity toward the OER/ORR, compared to pure ZnO.
) facets. Quite the contrary, the OER study showed that the introduction of Fe3+ ions into the ZnO crystal structure resulted in enhanced catalytic activity of all ZnO:Fe samples, compared to pure ZnO, probably due to the modified binding energy and an optimized band structure. With the maximal current density of 1.066 mA cm−2 at 2.216 V vs. RHE, an onset potential of 1.856 V vs. RHE, and the smallest potential difference between the OER and ORR (ΔE = 1.58 V), ZnO:10Fe may be considered a promising bifunctional catalyst toward the OER/ORR in moderately alkaline solution. This study demonstrates that the electrocatalytic activity of ZnO:Fe strongly depends on the defect chemistry and consequently the band structure. Along with providing fundamental insight into the electrocatalytic activity of ZnO:Fe, the study also indicates an optimal stoichiometry for enhanced bifunctional activity toward the OER/ORR, compared to pure ZnO.


 Please wait while we load your content...
Please wait while we load your content...