Triangulenes and theirs ions: reaching the limits of Clar's rule†
Abstract
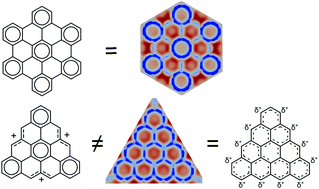
The triangulenes and their closed-shell ions are a family of polycyclic aromatic hydrocarbons (PAH) that have possible applications in fields as different as spintronics or catalysis. However, the electron delocalization in such systems is not well understood because there are several differences to classical PAHs. We found that the triangulene cations are always more delocalized than the radicals or the anions, independently of the π e− count. Contrary to any other PAHs, the π e− of triangulenes and their ions are delocalized throughout the whole molecule and are even more delocalized than acenes. The π sextet aromaticity does not play a central role in the stabilization of triangulenes like for other PAHs. Interestingly, neither the radicals nor the ions follow Clar's rule, which makes them a unique type of PAH.


 Please wait while we load your content...
Please wait while we load your content...