Rectification of bipolar nanopores in multivalent electrolytes: effect of charge inversion and strong ionic correlations
Abstract
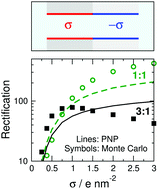
Bipolar nanopores have powerful rectification properties due to the asymmetry in the charge pattern on the wall of the nanopore. In particular, bipolar nanopores have positive and negative surface charges along the pore axis. Rectification is strong if the radius of the nanopore is small compared to the screening length of the electrolyte so that both cations and anions have depletion zones in the respective regions. The depths of these depletion zones is sensitive to sign of the external voltage. In this work, we are interested in the effect of the presence of strong ionic correlations (both between ions and between ions and surface charge) due to the presence of multivalent ions and large surface charges. We show that strong ionic correlations cause leakage of the coions, a phenomenon that is absent in mean field theories. In this modeling study, we use both the mean-field Poisson–Nernst–Planck (PNP) theory and a particle simulation method, Local Equilibrium Monte Carlo (LEMC), to show that phenomena such as overcharging and charge inversion cannot be reproduced with PNP, while LEMC is able to produce nonmonotonic dependence of currents and rectification as a function of surface charge strength.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...