A statistical thermodynamics view of electron density polarisation: application to chemical selectivity
Abstract
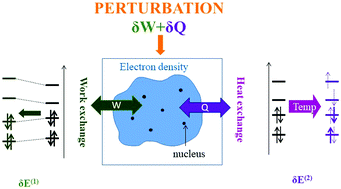
A fundamental link between conceptual density functional theory and statistical thermodynamics is herein drawn, showing that intermolecular electrostatic interactions can be understood in terms of effective work and heat exchange. From a more detailed analysis of the heat exchange in a perturbation theory framework, an associated entropy can be subsequently derived, which appears to be a suitable descriptor for the local polarisability of the electron density. A general rule of thumb is evidenced: the more the perturbation can be spread, both through space and among the excited states, the larger the heat exchange and entropy.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...