How Parkinson's disease-related mutations disrupt the dimerization of WD40 domain in LRRK2: a comparative molecular dynamics simulation study†
Abstract
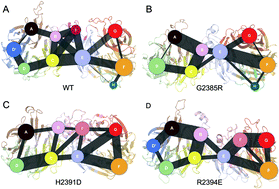
The multidomain kinase enzyme leucine-rich-repeat kinase 2 (LRRK2), activated through a homodimerization manner, has been identified as an important pathogenic factor in Parkinson's disease (PD), the second most common neurodegenerative disease wordwide. The Trp-Asp-40 (WD40) domain, located in the C-terminal LRRK2, harbours one of the most frequent PD-related variants, G2385R. However, the detailed dynamics of WD40 during LRRK2 dimerization and the underlying mechanism through which the pathogenic mutations disrupt the formation of the WD40 dimer have remained elusive. Here, microsecond-scale molecular dynamics simulations were employed to provide a mechanistic view underlying the WD40 dimerization and unveil the structural basis by which the interface-based mutations G2385R, H2391D and R2394E compromise the corresponding process. The simulation results identified important residues, D2351, R2394, E2395, R2413, and R2443, involved in establishing the complex binding network along the dimerization interface, which was significantly weakened in the presence of interfacial mutations. A “sag–bulge” model was proposed to explain the unfavorable dimer formation in the mutant systems. In addition, mutations altered the community configuration in the wild-type system in which inter-monomeric interplay is prominent, leading to the destabilization of the WD40 dimer under mutation.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...