The interaction strength of an intrinsically disordered protein domain with its binding partner is little affected by very different cosolutes
Abstract
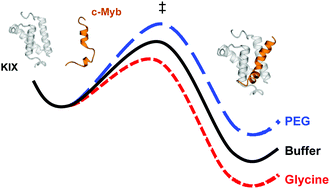
A common feature of intrinsically disordered proteins (IDPs) is a disorder-to-order transition upon binding to other proteins, which has been tied to multiple benefits, including accelerated association rates or binding with low affinity, yet high specificity. Given the balanced equilibrium concentrations of folded and unfolded state of an IDP we asked the question if changes in the chemical environment, such as the presence of osmolytes or crowding agents, have a strong influence on the interaction of an IDP. Here, we demonstrate the impact of cosolutes on the interaction of the intrinsically disordered transcription factor c-Myb and its binding partner, the kinase-inducible interaction domain (KIX) of the CREB-binding protein. Temperature jump relaxation kinetics and microscale thermophoresis were employed in order to quantify the rate constants and the binding affinity of the c-Myb/KIX complex, respectively, in the presence of various cosolutes. We find the binding free energy of the c-Myb/KIX complex only marginally modulated by cosolutes, whereas the enthalpy and entropy of the interaction are very sensitive to the respective solvent conditions. For different cosolutes we observe substantial changes in enthalpy, both favorable and unfavorable, which are going with entropy changes largely compensating the enthalpy effects in each case. These characteristics might reflect a potential mechanism by which c-Myb offsets changes in the physico-chemical environment to maintain a roughly unaltered binding affinity.


 Please wait while we load your content...
Please wait while we load your content...