Nanoscale kinetics of amorphous calcium carbonate precipitation in H2O and D2O†
Abstract
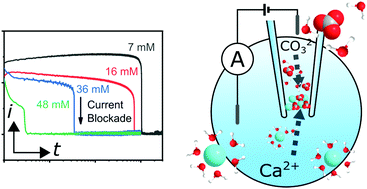
Calcium carbonate (CaCO3) is one of the most well-studied and abundant natural materials on Earth. Crystallisation of CaCO3 is often observed to proceed via an amorphous calcium carbonate (ACC) phase, as a precursor to more stable crystalline polymorphs such as vaterite and calcite. Despite its importance, the kinetics of ACC formation have proved difficult to study, in part due to rapid precipitation at moderate supersaturations, and the instability of ACC with respect to all other polymorphs. However, ACC can be stabilised under confinement conditions, such as those provided by a nanopipette. This paper demonstrates electrochemical mixing of a Ca2+ salt (CaCl2) and a HCO3− salt (NaHCO3) in a nanopipette to repeatedly and reversibly precipitate nanoparticles of ACC under confined conditions, as confirmed by scanning transmission electron microscopy (STEM). Measuring the current as a function of applied potential across the end of the nanopipette and time provides millisecond-resolved measurements of the induction time for ACC precipitation. We demonstrate that under conditions of electrochemical mixing, ACC precipitation is extremely fast, and highly pH sensitive with an apparent third order dependence on CO32− concentration. Furthermore, the rate is very similar for the equivalent CO32− concentrations in D2O, suggesting that neither ion dehydration nor HCO3− deprotonation represent significant energetic barriers to the formation of ACC. Finite element method simulations of the electrochemical mixing process enable the supersaturation to be estimated for all conditions and accurately predict the location of precipitation.


 Please wait while we load your content...
Please wait while we load your content...