Can the roles of polar and non-polar moieties be reversed in non-polar solvents?†
Abstract
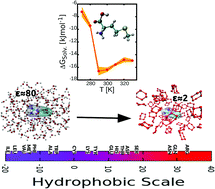
Using thermodynamic integration, we study the solvation free energy of 18 amino acid side chain equivalents in solvents with different polarities, ranging from the most polar water to the most non-polar cyclohexane. The amino acid side chain equivalents are obtained from the 20 natural amino acids by replacing the backbone part with a hydrogen atom, and discarding proline and glycine that have special properties. A detailed analysis of the relative solvation free energies suggests how it is possible to achieve a robust and unambiguous hydrophobic scale for the amino acids. By discriminating the relative contributions of the entropic and enthalpic terms, we find strong negative correlations in water and ethanol, associated with the well-known entropy–enthalpy compensation, and a much reduced correlation in cyclohexane. This shows that in general the role of the polar and non-polar moieties cannot be reversed in a non-polar solvent. Our findings are compared with past experimental as well as numerical results, and may shed additional light on the unique role of water as a biological solvent.


 Please wait while we load your content...
Please wait while we load your content...