Differences in thermal structural changes and melting between mesophilic and thermophilic dihydrofolate reductase enzymes†
Abstract
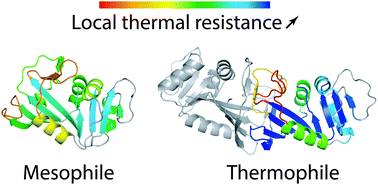
A key aspect of life's evolution on Earth is the adaptation of proteins to be stable and work in a very wide range of temperature conditions. A detailed understanding of the associated molecular mechanisms would also help to design enzymes optimized for biotechnological processes. Despite important advances, a comprehensive picture of how thermophilic enzymes succeed in functioning under extreme temperatures remains incomplete. Here, we examine the temperature dependence of stability and of flexibility in the mesophilic monomeric Escherichia coli (Ec) and thermophilic dimeric Thermotoga maritima (Tm) homologs of the paradigm dihydrofolate reductase (DHFR) enzyme. We use all-atom molecular dynamics simulations and a replica-exchange scheme that allows to enhance the conformational sampling while providing at the same time a detailed understanding of the enzymes’ behavior at increasing temperatures. We show that this approach reproduces the stability shift between the two homologs, and provides a molecular description of the denaturation mechanism by identifying the sequence of secondary structure elements melting as temperature increases, which is not straightforwardly obtained in the experiments. By repeating our approach on the hypothetical TmDHFR monomer, we further determine the respective effects of sequence and oligomerization in the exceptional stability of TmDFHR. We show that the intuitive expectation that protein flexibility and thermal stability are correlated is not verified. Finally, our simulations reveal that significant conformational fluctuations already take place much below the melting temperature. While the difference between the TmDHFR and EcDHFR catalytic activities is often interpreted via a simplified two-state picture involving the open and closed conformations of the key M20 loop, our simulations suggest that the two homologs’ markedly different activity temperature dependences are caused by changes in the ligand–cofactor distance distributions in response to these conformational changes.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...