Structural rearrangements as relaxation pathway for molecular negative ions formed via vibrational Feshbach resonance†
Abstract
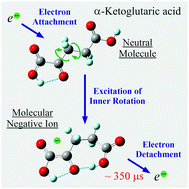
The low-energy (0–15 eV) resonance electron interaction with two organic acids, oxaloacetic and α-ketoglutaric, is studied under gas-phase conditions using dissociative electron attachment spectroscopy. The most unexpected observation is the long-lived (microseconds) molecular negative ions formed by thermal electron attachment via the vibrational Feshbach resonance mechanism in both compounds. Unlike oxaloacetic acid, for which only one slow (microseconds) dissociative decay is detected, as many as five metastable negative ions are observed for α-ketoglutaric acid. These results are analyzed using density functional theory calculations and estimations of electron affinity using the experimental electron detachment times. The results are of considerable interest for understanding the fundamental mechanisms responsible for the dynamics of highly excited negative ions and the transformation pathways of biologically relevant molecules stimulated by excess electron attachment.


 Please wait while we load your content...
Please wait while we load your content...