Lithium, sodium and magnesium ion conduction in solid state mixed polymer electrolytes†
Abstract
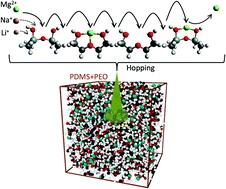
Alkali and alkaline earth metal-ion batteries are currently among the most efficient electrochemical energy storage devices. However, their stability and safety performance are greatly limited when used with volatile organic liquid electrolytes. A solid state polymer electrolyte is a prospective solution even though poor ionic conductivity at room temperature remains a bottleneck. Here we propose the mixing of two similar polymer matrices, poly(dimethyl siloxane) and poly(ethylene oxide), to address this challenge. The resulting electrolyte matrix is denser and significantly improves room-temperature ionic conductivity. Ab initio analyses of the reaction between the cations and the polymers show that oxygen sites act as entrapment sites for the cations and that ionic conduction likely occurs through hopping between adjacent oxygen sites. Molecular dynamics simulations of the dynamics of both polymers and the dynamics of the polymer mix show that the more frequent and more pronounced molecular vibrations of the polymer mix are likely responsible for reducing the time between two consecutive oxygen entrapments, thereby speeding up the conduction process. This hypothesis is experimentally validated by the practically useful ionic conductivity (σ ≈ 10−4 S cm−1 at 25 °C) and the improved safety parameters exhibited by a transparent flexible multi-cation (Li+, Na+ and Mg2+) conducting solid channel made up of the above mixed polymer system.


 Please wait while we load your content...
Please wait while we load your content...