A revisit of the bond valence model makes it universal
Abstract
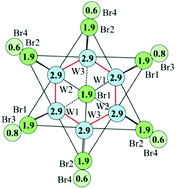
The use of simple, intuitive equations to correlate the geometry of crystal structures with electron descriptors of chemical bonds and material structural stability is a great advantage of the Bond Valence Model (BVM), which is based on Pauling's principles of bond order (BO) conservation and exponential BO/bond length relationship. However, the high potential of BVM to be used as an important analytical tool was overlooked in recent inorganic chemistry due to its empirical character and serious restrictions for its application. Recent quantum chemistry data (BOs and electron densities at the bond critical points, ρc) enable us to establish the validity of the BVM to any type of chemical bonds, as well as a direct BO/ρc relationship. Such a BVM revisit overcomes most of the limitations anticipated previously for the model and thus makes it universal. This Perspective highlights the advance in model development, in particular its application to compounds with metal–metal bonds, which allows us to establish (i) a linear correlation between BOs and stretching force constants used as a measure of the bond strength and (ii) a quantitative description of the steric/electrostatic effects in cluster compounds enabling us to understand their nature and the influence of such effects on structural stability. Thus, using interatomic distances, the simple Pauling equation and empirical constants, it is possible to calculate effective BOs and predict stretching force constants and electron densities at the bond critical points in any complex compound, all of this at zero cost!
- This article is part of the themed collections: Quantum Theory: The Challenge of Transition Metal Complexes and PCCP Perspectives


 Please wait while we load your content...
Please wait while we load your content...