Exploring the role of vacancy defects in the optical properties of LiMgPO4†
Abstract
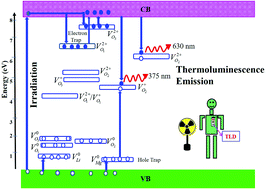
Linearity in dose response up to very high radiation doses and sufficient sensitivity to even low radiation doses are extremely important for the measurement of radiation dose in the field of radiation technology, ranging from medical to industrial applications. Olivine type LiMgPO4 has been shown immense interest as a phosphor material in the fields of thermoluminescence and optically stimulated luminescence dosimetry. In the present study, we have explored the role of different vacancy defects in the optical properties of LiMgPO4 aiming at enhancing its sensitivity for the measurement of radiation dose. For this purpose, we have systematically investigated the electronic structure of LiMgPO4 in the absence and presence of various vacancy defects using density functional theory as a tool. The present study considers all possible vacancy defects including neutral, charged and mixed lattice vacancy defects in LiMgPO4. To find the most energetically favourable vacancy defect, we have compared the defect formation energy of all the vacancy defects. We have also calculated vacancy formation energy in different chemical environments to investigate how the formation of different types of vacancy defect can be controlled by tuning the chemical environment. Finally, the origin of the different optical properties of LiMgPO4 has been explained by using a possible mechanism based on our detailed electronic structure calculations. Thus, the present study is believed to provide valuable insight for the development of materials with improved features for the measurement of radiation dose.


 Please wait while we load your content...
Please wait while we load your content...