Effect of crystallite size on the phase transition behavior of heterosite FePO4†
Abstract
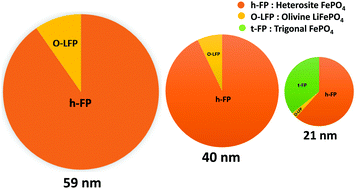
For advanced lithium-ion battery technology, olivine-based cathodes are considered to be the most dominant and technologically recognized materials. The extraction of lithium ions from olivine LiFePO4 results in the two-phase mixture with heterosite FePO4 exhibiting a deintercalation potential of 3.45 V vs. Li+/Li over a wide range of lithium content. Here, we report the synthesis and characterization of chemically deintercalated heterosite FePO4 with varying crystallite sizes using different analytical techniques. The decrease in the crystallite size of heterosite FePO4 leads to an increase in the lattice parameters including the unit cell volume. The characteristic behavior in the structural properties of heterosite FePO4 shows a strong dependency on the crystallite size which is correlated with the change in the chemical bonding. The volume expansion of the nano-sized heterosite FePO4 with respect to the bulk counterpart is suggested to be a direct consequence of reduced hybridization between the Fe3d and O2p states. Furthermore, the combined X-ray diffraction and Mössbauer spectroscopic studies reveal the appearance of a new phase namely trigonal FePO4 at the lower crystallite sizes due to the enhanced surface energy kinetics. We also find that the observed trigonal FePO4 phase is more magnetically active than the paramagnetic olivine FePO4. For the unique structural advantage of the heterosite phase as an electrode material, the change in bonding characteristics is very useful and can have strong implications on the electronic properties of heterosite FePO4 at the nanoscale level.


 Please wait while we load your content...
Please wait while we load your content...