Spectroscopic evidence for intact carbonic acid stabilized by halide anions in the gas phase†
Abstract
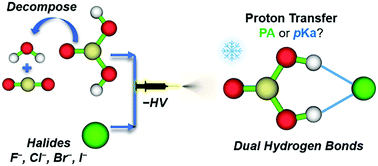
This work shows elusive carbonic acid being effectively stabilized in the gas phase by interacting with halide anions X− (X = F, Cl, Br, and I). The formed H2CO3·X− complexes, characterized by negative ion photoelectron spectroscopy and ab initio calculations, all contain intact trans–trans carbonic acid binding onto the respective halide via two identical strong ionic O–H⋯X− hydrogen bonds. For X = Cl, Br, and I, the complex spectra exhibit the corresponding X− signature by simply shifting to the higher binding energy side, while an extremely 2 eV wide broader band is observed for X = F. This spectroscopic evidence indicates that an excess electron is removed from each halide in the former case, while a proton is transferred from carbonic acid to fluoride upon electron detachment for the latter. The above H2CO3·X− structures as well as those of the previously studied H2SO4·X− along the homologous halogen series cannot be explained using the proton affinity (PA) argument. Instead, a qualitative correlation is found between these structural motifs and the constituent acid pKa values, strongly suggesting that pKa is a more suitable factor to predict correct acid–base chemistry between these diprotic oxyacids and halides.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...