Boosting water oxidation activity by tuning the proton transfer process of cobalt phosphonates in neutral solution†
Abstract
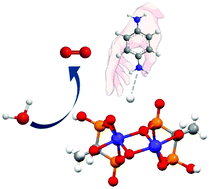
Water oxidation is a vital step in both natural and artificial photosynthetic processes. However, the effect of second coordination sphere for efficient oxygen evolution electrocatalysts has rarely been studied, becoming a bottleneck in many energy-related issues. In this article, the cobalt phosphonate (NH3C6H4NH3)Co2(hedpH)2·H2O (Co-PDA) displayed decent electrocatalytic water oxidation activity in 50 mM PBS solution (pH 7.0), comparable to the activity of state-of-the-art IrO2. Moreover, it exhibited a 160 mV lower onset potential and 6 times higher TOF than those of the counterpart, (NH4)2Co2(hedpH)2 (Co-NH4+), which existed with the same Co active center, while surrounded by different ligands. The related mechanistic studydemonstrates that the ligand in Co-PDA would benefit the proton-coupled electron transfer (PCET) processes and the formation of high valence state Co(IV).


 Please wait while we load your content...
Please wait while we load your content...