Theoretical investigation of the dissociation chemistry of formyl halides in the gas phase†
Abstract
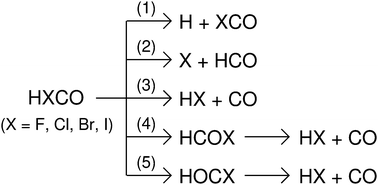
Halogen substituted analogues of formaldehyde, HXCO (X = F, Cl, Br, and I), play a crucial role in the degradation of stratospheric ozone. Several spectroscopic and quantum chemistry investigations of the dissociation chemistry of formyl halides have been reported in the literature. Due to their importance in combustion and atmospheric chemistry, we investigated the gas phase dissociation of formyl halides using electronic structure theory, direct chemical dynamics simulations, and Rice–Ramsperger–Kassel–Marcus rate constant calculations. Chemical dynamics simulations were performed using density functional B3LYP/6-31G* theory with suitable effective core potentials for the halogen atoms. Simulations showed multiple pathways and mechanisms for the dissociation of formyl halides. The major reaction products were HX + CO which formed via direct and indirect pathways. Trajectory lifetime distribution calculations indicated non-statistical dissociation dynamics.


 Please wait while we load your content...
Please wait while we load your content...