Does the photoredox reaction affect the photorelease of anthraquinone protected benzaldehyde? A time-resolved spectroscopic study†
Abstract
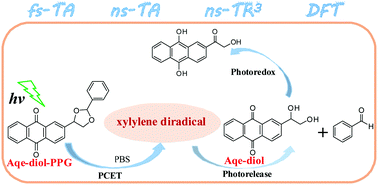
In this study, combined time-resolved spectroscopies of femtosecond transient absorption, nanosecond transient absorption, and nanosecond time-resolved resonance Raman spectroscopies as well as DFT calculations were performed to unravel the photorelease reaction mechanism of anthraquinon-2-ylethyl-1,2-diol protected carbonyl compound (Aqe-diol-PPG). It was shown that the triplet state of Aqe-diol-PPG underwent a PCET process to form a xylylene intermediate. After the C–O bond cleavage, a diradical intermediate was generated, which further released the protected benzaldehyde with the by-product Aqe-diol. A comparison work suggested that Aqe-diol underwent a further photoredox reaction in aqueous solutions.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...