Catalysis effect on CO2 methanation using MgH2 as a portable hydrogen medium†
Abstract
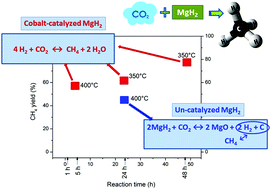
The feasibility of the reduction of CO2 to CH4 employing MgH2 in the presence and absence of cobalt as a catalyst was investigated for the first time, exploring different non-independent reaction conditions such as the grade of microstructural refinement, the molar ratio MgH2 : CO2, reaction time and temperature. For the un-catalyzed process a methane yield of 44.6% was obtained after 24 h of thermal treatment at 400 °C employing a molar ratio of 2 : 1, through a methanation mechanism that involves the direct reduction of CO2 and the generation of CH4via C as an intermediary. For the MgH2 catalyzed process a methane yield of 78% was achieved by heating at 350 °C for 48 h, 4 : 1 being the optimal molar ratio. The global mechanism corresponds to a Sabatier process favored by Co as an active catalyst, together with the reverse water gas shift reaction followed by methanation of CO in the presence of steam. On account of the fact that it was proved that the use of the catalyst allows lowering the operational temperature without collapsing the methane yield, this research provides interesting insight into a thermochemical method for CO2 reduction to CH4 employing a solid hydrogen storage medium as an H2 source.


 Please wait while we load your content...
Please wait while we load your content...