Conformational preferences of TEMPO type radicals in complexes with cyclodextrins revealed by a combination of EPR spectroscopy, induced circular dichroism and molecular modeling†
Abstract
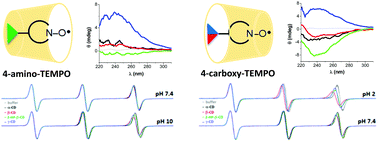
Electron paramagnetic resonance (EPR) spectroscopy is the main tool for evidencing the formation of inclusion complexes of cyclodextrins with paramagnetic guests, based on changes in the EPR parameters. In-depth information on complexation can only be obtained by a combination of physico-chemical methods. Herein we report on the interaction of three TEMPO (2,2,6,6-tetramethylpiperidine 1-oxyl) type radicals with cyclodextrins by collecting and analysing data provided experimentally by EPR and circular dichroism spectroscopies and theoretically by density functional theory and molecular docking. The study focused on the pH influence on the complexation of three paramagnetic probes with cyclodextrins. The EPR spectra revealed that the type and protonation state of the substituent linked to the TEMPO structure influences the affinity of the paramagnetic group for the cyclodextrin cavity. Neutral radical species favour stronger association with cyclodextrins and inclusion of the nitroxide group into the cavity, especially in the case of 4-carboxy-TEMPO. Induced circular dichroism signals of neutral species varied in sign and intensity as a function of substituent and cyclodextrin type. Density functional theory and molecular docking results supported the experimental data regarding the conformational preferences of TEMPO radicals in complexes with cyclodextrins.


 Please wait while we load your content...
Please wait while we load your content...