High pressure single-molecule FRET studies of the lysine riboswitch: cationic and osmolytic effects on pressure induced denaturation†
Abstract
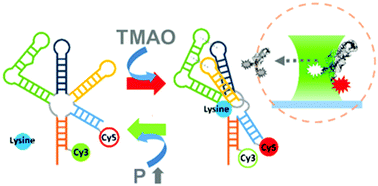
Deep sea biology is known to thrive at pressures up to ≈1 kbar, which motivates fundamental biophysical studies of biomolecules under such extreme environments. In this work, the conformational equilibrium of the lysine riboswitch has been systematically investigated by single molecule FRET (smFRET) microscopy at pressures up to 1500 bar. The lysine riboswitch preferentially unfolds with increasing pressure, which signals an increase in free volume (ΔV0 > 0) upon folding of the biopolymer. Indeed, the effective lysine binding constant increases quasi-exponentially with pressure rise, which implies a significant weakening of the riboswitch–ligand interaction in a high-pressure environment. The effects of monovalent/divalent cations and osmolytes on folding are also explored to acquire additional insights into cellular mechanisms for adapting to high pressures. For example, we find that although Mg2+ greatly stabilizes folding of the lysine riboswitch (ΔΔG0 < 0), there is negligible impact on changes in free volume (ΔΔV0 ≈ 0) and thus any pressure induced denaturation effects. Conversely, osmolytes (commonly at high concentrations in deep sea marine species) such as the trimethylamine N-oxide (TMAO) significantly reduce free volumes (ΔΔV0 < 0) and thereby diminish pressure-induced denaturation. We speculate that, besides stabilizing RNA structure, enhanced levels of TMAO in cells might increase the dynamic range for competent riboswitch folding by suppressing the pressure-induced denaturation response. This in turn could offer biological advantage for vertical migration of deep-sea species, with impacts on food searching in a resource limited environment.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...