Hydrogen bond, ring tension and π-conjugation effects: methyl and vinyl substitutions dramatically change the photodynamics of Criegee intermediates†
Abstract
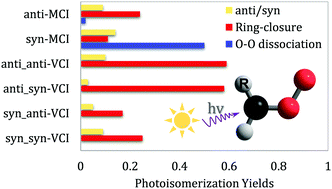
The substitution effect in chemistry is a concept that is probably too common to mention, while for a molecule with an elusive electronic structure, substitution can introduce an unusual effect that dramatically tunes the chemical process. To reveal the substitution effects on the photodynamics of Criegee Intermediates (CIs), we carried out the multireference CASSCF trajectory surface-hopping (TSH) molecular dynamics and CASPT2 electronic-structure calculations for a methyl-substituted CI (MCI) and a vinyl-substituted CI (VCI). The results show that for different substituents, the hydrogen bond, ring tension and π-conjugation not only alter the relative stabilities of the conformers/configurations, but also dramatically change the photo-induced channel of CIs. For an anti-MCI, the dominant channel starting from the S1 state is the ring-closure process leading to dioxirane, while in the syn configuration, the intramolecular CH⋯O hydrogen bond hinders the rotation around the C–O bond and thus leads to a high yield of in-plane O–O dissociation towards acetaldehyde (X1A′) and the O(1D) atom. In a VCI with an unsaturated substituent, the π-conjugation greatly strengthens the O–O bond and therefore no O–O dissociation is observed in all configurations. In addition, the CH⋯O hydrogen bond in the syn(CO)-VCI further stabilizes the S1-state intermediates and makes them less reactive; in contrast, isomerization to dioxirane becomes the globally dominant channel in the anti(CO)-VCI. The dramatic substitution effects by saturated and unsaturated substituents on CIs found here will deepen the understanding of Criegee-intermediate chemistry.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...