Absorption spectra of pyruvic acid in water: insights from calculations for small hydrates and comparison to experiment†
Abstract
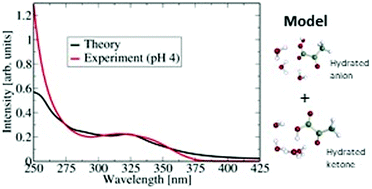
Pyruvic acid is abundant in the atmosphere and in seawater, being a decay product of living organisms. Although very small in size (10 atoms), pyruvic acid exhibits conformational complexity in the gas phase and in solution, which is reflected in the UV spectrum. The gas phase UV spectrum of pyruvic acid differs from the spectrum of pyruvic acid in water. The main atmospherically relevant absorption peak in the gas phase is blue shifted by about 0.43 eV (40 nm difference in the peak location) in water. The origin of the blue shift has not been established thus far. This paper aims at a microscopic understanding of the absorption spectrum of pyruvic acid in aqueous media by a combined experimental and theoretical approach. 1H NMR experiments were performed to reveal the contribution of the different conformers in solution as a function of pH. Computationally, hydrates of sizes up to 5 water molecules using two different species of pyruvic acid, the neutral acid and the anionic form were considered. Vertical excitation energies using the ADC(2) method (algebraic-diagrammatic construction through second order) of these structures provide insights into the blue shift of the atmospherically relevant absorption peak. Additionally, molecular dynamics simulation on MP2 (Møller–Plesset perturbation theory) ground state of small clusters of pyruvic acid with four water molecules were calculated and used in computing the vertical excitation spectrum along the dynamics. This is found to describe very accurately the experimental spectrum. Overall, the results show that small hydrate models including the roles of both neutral and deprotonated speciated forms provide a good quantitative description and a microscopic interpretation of the experimental spectrum of pyruvic acid in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...