NO disproportionation over defective 1T′-MoS2 monolayers†
Abstract
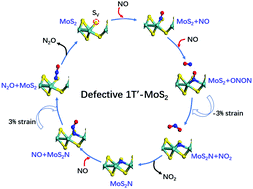
The MoS2 monolayers are usually created with vacancies (most likely missing S atoms). At an S vacancy, the exposed and under-coordinated Mo atoms become reactive and can strongly bind to small molecules. Here, by using first-principles calculations, it is proved that 1T′-MoS2 monolayers are an efficient catalyst for NO disproportionation. The reaction starts with NO adsorptions at the exposed Mo atoms. Later the incoming NO molecules react with the ones already adsorbed to give NO2 molecules, which readily desorb. The remaining N-doped MoS2 sheets can then easily react with NO molecules to produce N2O, and can be heated to desorb them. Thus, the defective 1T′-MoS2 monolayers are recovered and the catalytic cycle is completed. The NO2 formation step has a relatively high activation barrier of 1.58 eV, but it can be lowered to 0.19 or 0.56 eV by applying biaxial −3% or 3% strain, respectively. The reaction mechanism is totally different from those catalyzed by metal-centered catalysts (complexes, clusters, or metal–organic frameworks), which feature the N2O formation as the rate-limiting step and the NO2 in the metal–nitrite complexes cannot be released. This work paves the way for strain engineering two-dimensional (2D) materials into efficient NO disproportionation catalysts.


 Please wait while we load your content...
Please wait while we load your content...