Impact of the olefin structure on the catalytic cycle and decomposition rates of Hoveyda–Grubbs metathesis catalysts†
Abstract
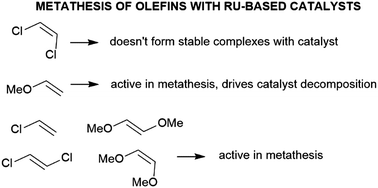
A relatively fast degradation of ruthenium catalysts in the presence of selected olefins, and ethylene in particular, is one of the bottlenecks in their use in metathesis reactions. Here we explore the structure–activity relationships between the rate of degradation of Hoveyda–Grubbs catalysts and the structure of olefins by means of DFT calculations. We show that (Z)-1,2-dichloroethene can't form stable complexes with a 14-electron active complex due to a strong inductive electron withdrawal effect. Hoveyda–Grubbs catalysts can be, however, used to convert (Z)-1,2-dichloroethene to (E)-1,2-dichloroethene due to differences in crucial barriers in the catalytic cycle for E/Z isomers. Hoveyda–Grubbs catalysts in the presence of both isomers of 1,2-dimethoxyethene and 1,2-dichloroethene are predicted to be very stable in the unproductive metathesis, while for monosubstituted olefins the methoxyethene presence gives relatively low barriers for crucial degradation transition states and can readily undergo decomposition.


 Please wait while we load your content...
Please wait while we load your content...