Non-specific DNA-driven quinary interactions promote structural transitions in proteins†
Abstract
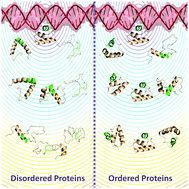
The nature and distribution of charged residues on the surface of proteins play a vital role in determining the binding affinity, selectivity and kinetics of association to ligands. When it comes to DNA-binding domains (DBDs), these functional features manifest as anisotropic distribution of positively charged residues on the protein surface driven by the requirement to bind DNA, a highly negatively charged polymer. In this work, we compare the thermodynamic behavior of nine different proteins belonging to three families – LacR, engrailed and Brk – some of which are disordered in solution in the absence of DNA. Combining detailed electrostatic calculations and statistical mechanical modeling of folding landscapes at different distances and relative orientations with respect to DNA, we show that non-specific electrostatic interactions between the protein and DNA can promote structural transitions in DBDs. Such quinary interactions that are strictly agnostic to the DNA sequence induce varied behaviors including folding of disordered domains, partial unfolding of ordered proteins and (de-)population of intermediate states. Our work highlights that the folding landscape of proteins can be tuned as a function of distance from DNA and hints at possible reasons for DBDs exhibiting complex kinetic-thermodynamic behaviors in the absence of DNA.


 Please wait while we load your content...
Please wait while we load your content...