Reaction mechanism of N-cyclopropylglycine oxidation by monomeric sarcosine oxidase†
Abstract
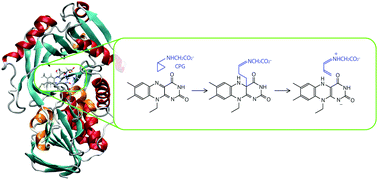
Monomeric sarcosine oxidase (MSOX) is a fundamental – yet one of the simplest – member of a family of flavoenzymes able to catalyze the oxidation of sarcosine (N-methylglycine) and other secondary amines. MSOX is one of the best characterized members of the amine oxidoreductases (AOs), however, its reaction mechanism is still controversial. A single electron transfer (SET) process was suggested on the basis of studies with N-cyclopropylglycine (CPG), although a hydride transfer mechanism would be more consistent in general for AOs. To shed some light on the detailed reaction mechanisms of CPG in MSOX, we performed hybrid quantum mechanical/molecular mechanical (QM/MM) simulations. We found that the polar mechanism is energetically the most favorable. The free energy profile indicates that the first rate-limiting step is the CPG binding to the flavin ring which simultaneously proceeds with the ring-opening of the CPG cyclopropyl group. This reaction step of the CPG adduct formation corresponds to the nucleophilic attack of the cyclopropyl group (C3 atom) to the flavin ring (C4a atom), whereas the expected radical species formation in the SET mechanism was not observed. The following inactivated species, which accumulates during the CPG oxidation in MSOX, can be ascribed to an imine state, and not an enamine state, on the basis of the computed UV/Vis spectra. The conformation of CPG was found to be crucial for reactions following the CPG adduct formation.


 Please wait while we load your content...
Please wait while we load your content...