Radical⋯radical chalcogen bonds: CSD analysis and DFT calculations†
Abstract
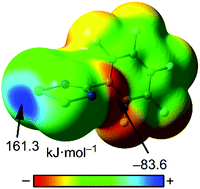
This manuscript reports a combination of crystallographic analysis (Cambridge Structural Database) and theoretical DFT calculations in chalcogen bonding interactions involving radicals in both the Ch bond (ChB) donor and acceptor. As a radical ChB acceptor (nucleophile) we have used benzodithiazolyl radical (BDTA) and as Ch bond donors (electrophile) we have used dithiadiazolyl and diselenadiazolyl radicals of the general formula p-X-C6F4–CNChChN (Ch = S, and Se). We have evaluated how the para substituent (X) affects the interaction energy, spin density and charge/spin transfer from the electron rich BDTA radical to the electron poor dichalcogenadiazolyl ring. The ability of the latter rings to form ChBs in the solid state has been examined by a comprehensive search in the CSD; several cases are used to exemplify the preferred geometric features of the complexes and they are compared with the theory. The molecular surface electrostatic potentials calculated for these ChB donors allow for a very precise rationalization of the self-assembly motifs most frequently adopted in the crystalline state and of their relative robustness.


 Please wait while we load your content...
Please wait while we load your content...