On-surface synthesis of extended linear graphyne molecular wires by protecting the alkynyl group†
Abstract
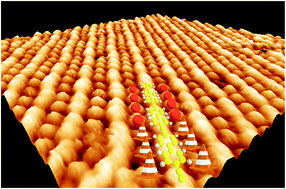
In this paper we report on the use of an Ullmann-like aryl halide homocoupling reaction to obtain long Graphyne Molecular Wires (GY MWs) organized in dense, ordered arrays. Instead of using highly reactive terminal alkynes, we resort to a precursor wherein the acetylenic functional group is internal, namely protected by two phenyl rings, each bearing a Br atom in the para position to allow for linear homocoupling. In addition, two further factors concur with the production of dense and highly ordered arrays of very long GY MWs, namely the geometric compatibility between the substrate and both the organometallic intermediates and the final polymeric products of the synthesis, coupled with the presence of surface-adsorbed bromine atoms separating the MWs, which minimize inter-wire cross-linking secondary reactions.


 Please wait while we load your content...
Please wait while we load your content...