Ligand architectural effect on coordination, bonding, interaction, and selectivity of Am(iii) and Ln(iii) ions with bitopic ligands: synthesis, solvent extraction, and DFT studies†
Abstract
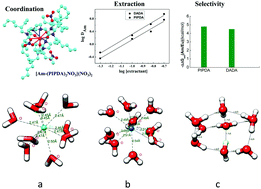
The isolation of Am(III) ion from Ln(III) ions is very crucial for the safe disposal of nuclear wastes and thus, studies are being continuously pursued to accomplish this goal. In view of this, herein, a new conformationally rigid bitopic ligand, N,N,N′,N′-tetra(2-ethylhexyl)piperazine-di-methylenecarboxamide (PIPDA) has been synthesized and studied for the separation of Am(III) from Ln(III) ions. The effect of structural rigidification on the selectivity of Am(III) over Ln(III) was compared with an open chain flexible compound, namely, N,N,N′,N′-tetra(2-ethylhexyl)-3,6-(N′′,N′′′-dibutyl)diaza-octane-1,8-diamide (DADA). Two oxygen atoms of the diamide moiety seem to be responsible for controlling the metal ion extraction ability of PIPDA, whereas two nitrogen atoms of the piperazine moiety most probably dictate the separation factor between the Am(III) and Eu(III) ions in PIPDA. In addition, scalar relativistic density functional theory (DFT) in conjunction with Born–Haber thermodynamics was used herein to compliment the experimental selectivity. The experimentally observed preferential selectivity of PIPDA for Am(III) ion over the Ln(III) ion was corroborated by the computed extraction free energy, ΔGext. The covalent nature of bonding between the metal ions and the ligand was confirmed by analyzing the Mayer bond order and bond character analysis using the atom in molecule concept. Though the conformational rigidity of PIPDA gives stronger interaction than DADA, it does not offer a significant advantage over DADA in terms of the separation factor. The marginal increase in the separation factor for PIPDA over DADA might be attributed to the piperazine nitrogen and to the ligand architecture during complex formation.


 Please wait while we load your content...
Please wait while we load your content...