The interaction of two-dimensional α- and β-phosphorus carbide with environmental molecules: a DFT study†
Abstract
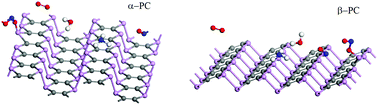
The recently fabricated two-dimensional phosphorus carbide (PC) has been proposed for application in different nanodevices such as nanoantennas and field-effect transistors. However, the effect of ambient molecules on the properties of PC and, hence, the productivity of PC-based devices is still unknown. Herein a first-principles investigation is performed to study the most structurally stable α- and β-PC allotropes upon their interaction with environmental molecules, including NH3, NO, NO2, H2O, and O2. It is predicted that NH3, H2O, and O2 are physisorbed on α- and β-PC while NO and NO2 may easily form a covalent bond with the PC. Importantly, NO and NO2 possess low adsorption energies on PC which compared to these on graphene and phosphorene. Moreover, both molecules are strong acceptors to PC with a giant charge transfer of ∼1 e per molecule. For all the considered molecules PC is found to be more sensitive compared to graphene and phosphorene. The present work provides useful insight into the effects of environmental molecules on the structure and electronic properties of α- and β-PC, which may be important for their manufacturing, storage, and application in gas sensors and electronic devices.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...