Reactions of radiation-induced electrons with carbon dioxide in inert cryogenic films: matrix tuning of the excess electron interactions in solids†
Abstract
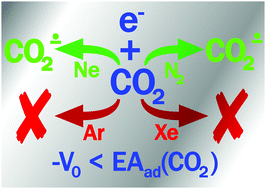
A single-electron reduction of carbon dioxide is supposed to be an important basic step in various processes, ranging from interstellar chemistry to photocatalytic transformations. In this work, we report an FTIR spectroscopic study on the reactions of carbon dioxide (12CO2 and 13CO2) with the radiation-induced excess electrons in deposited cryogenic matrices with different physical characteristics (Ne, N2, Ar, Xe) occurring at 6 K. The reaction was monitored by the observation of carbon dioxide radical anions. It was found that attachment of excess electrons to CO2 occurred in neon and nitrogen matrices, but not in argon and xenon. In the case of nitrogen, the formation of matrix-related cationic species (N4+˙ and NNCO+˙) was also observed. Since the CO2 molecules have a negative intrinsic electron affinity, it was suggested that the electron attachment to CO2 is controlled by the energy of excess electrons in the solid matrix, which is determined by the value of the corresponding conduction band bottom energy (V0). The implications of the obtained results are discussed.


 Please wait while we load your content...
Please wait while we load your content...