Multifunctionality of lanthanum–strontium manganite nanopowder†
Abstract
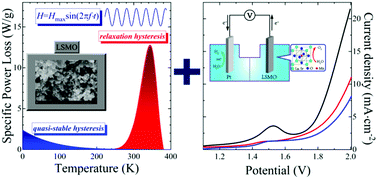
Manganites are multifunctional materials which are widely used in both technology and devices. In this article, new prospects of their use as nanoparticles for various types of applications are demonstrated. For that, the ferromagnetic nanopowder of La0.6Sr0.4MnO3 has been synthesized by the sol–gel method with a subsequent annealing at 700–900 °C. The crystal structure, phase composition and morphology of nanoparticles as well as magnetic, magnetothermal and electrocatalytic properties have been studied comprehensively. The critical sizes of superparamagnetic, single-domain, and multi-domain states have been determined. It has been established that an anomalously wide temperature range of magnetocaloric properties is associated with an additional contribution to the magnetocaloric effect from superparamagnetic nanoparticles. The maximum values of the specific loss power are observed in the relaxation hysteresis region near the magnetic phase transition temperature. The electrochemical stability and features of the decomposition of nanoparticles in 1 M KOH and Na2SO4 electrolytes have been determined. A decrease in the particle size contributes to an increase in electrocatalytic activity for overall water splitting. Magnetocaloric and electrocatalytic results of the work indicate the prospects for obtaining the possibility of changing the temperature regime of electrocatalysis using contactless heating or cooling.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...