The water trimer reaction OH + (H2O)3 → (H2O)2OH + H2O†
Abstract
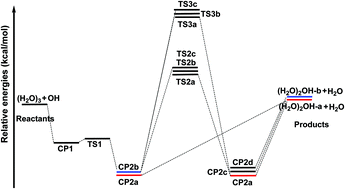
All important stationary points on the potential energy surface (PES) for the reaction OH + (H2O)3 → (H2O)2OH + H2O have been fully optimized using the “gold standard” CCSD(T) method with the large Dunning correlation-consistent cc-pVQZ basis sets. Three types of pathways were found. For the pathway without hydrogen abstraction, the barrier height of the transition state (TS1) is predicted to lie 5.9 kcal mol−1 below the reactants. The two major complexes (H2O)3⋯OH (CP1 and CP2a) are found to lie 6.3 and 11.0 kcal mol−1, respectively, below the reactants [OH + (H2O)3]. For one of the H-abstraction pathways the lowest classical barrier height is predicted to be much higher, 6.1 kcal mol−1 (TS2a) above the reactants. For the other H-abstraction pathway the barrier height is even higher, 15.0 (TS3) kcal mol−1. Vibrational frequencies and the zero-point vibrational energies connected to the PES are also reported. The energy barriers for the H-abstraction pathways are compared with those for the OH + (H2O)2 and OH + H2O reactions, and the effects of the third water on the energetics are usually minor (0.2 kcal mol−1).


 Please wait while we load your content...
Please wait while we load your content...